PROJECTS
Diabetes mellitus is a pandemic metabolic disorder characterized by chronic hyperglycemia. The pathogenic process is the result of insulin insufficiency. Insulin is a peptide hormone produced in the beta cells of the pancreatic islets. Current diabetes treatments strive for glycemic control but fail to solve the underlying beta cell defect. This approach has increased the life expectancy and quality of most patients with diabetes but has turned diabetes into a chronic disease.
Curative strategies should aim at restoring and protecting the functional beta cell mass which in turn would selfregulate a fine-tuned glucose homeostasis. Such alternative beta cell replacement therapies could either rely on exogenous delivery of beta cells through transplantation, or on endogenous repair of the endocrine pancreas through beta cell regeneration.
The main aim of the BENE lab is to (re)generate functional beta cells.
Generation and regeneration of beta cell mass
Beta cell regeneration is still a preclinical and experimental approach to diabetes therapy. Our understanding of the regenerative capacity of beta cells is limited and even more limited is our knowledge on mechanisms to generate more beta cells. Where do new beta cells come from? Proliferation of pre-existing beta cells? Differentiation from progenitor cells? Transdifferentiation of non-beta cells to beta(-like) cells?
In vivo
We approach this question by manipulating the islet micro-environment and studying the effect on the beta cell: either by decreasing or increasing the number of intra-islet endothelial cells or immune cells. The experimental models in which we perform these manipulations are islet transplantation, tissue damage, conditional gene overexpression (genetic, virus- or mRNA-mediated) or knockdown (genetic, virus- or siRNA-mediated)
In vitro
We try to generate new beta cells from non-beta cells using overexpression of key developmental transcription factors (virus- or mRNA mediated) and by chemical compounds found by high-throughput screening.
Project 1: Mechanisms behind generation of beta cells in injured pancreas (PDL)
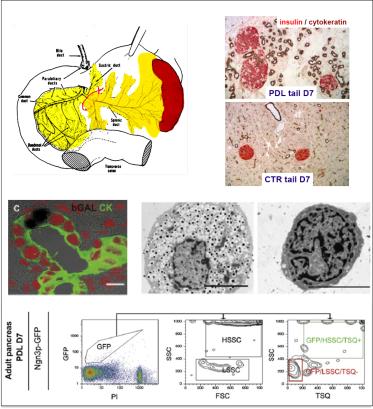
Diabetes is currently treated by drugs that support or partially replace beta cell function, but the disease cannot be cured at presence. A true regenerative therapy would greatly improve the quality of life for diabetes patients and could avert its costly long-term complications. One of the major challenges towards cure is the well-controlled generation of beta cells.
We developed experimental models that allow the investigation of mechanisms that increase the number of beta cells. One of these models, partial duct ligation (PDL), is based on a micro-surgical intervention that ligates the exocrine duct that drains the tail part of the pancreas and results in a doubling of the beta cell mass (Xu et al, Cell. 2008; Van de Casteele et al, Cell Death Dis. 2013; Van de Casteele et al, Diabetes. 2014). The increase in cell number is caused by the activation of facultative Neurog3+ progenitor cells as well as by proliferation of pre-existing and newly formed beta cells. We currently elaborate on the identification of the facultative progenitor cells and the cellular and molecular mechanisms that drive beta cell generation in the injured mouse pancreas using state of the art facilities for cell imaging, like light-sheet microscopy and for gene expression analysis, like single-cell transcriptomics. Our aim is to apply these differentiation and mitogenic signals to human pancreatic cells to induce facultative progenitor cell activation and beta cell proliferation.
Project 2: Designing a microenvironment for beta cell proliferation
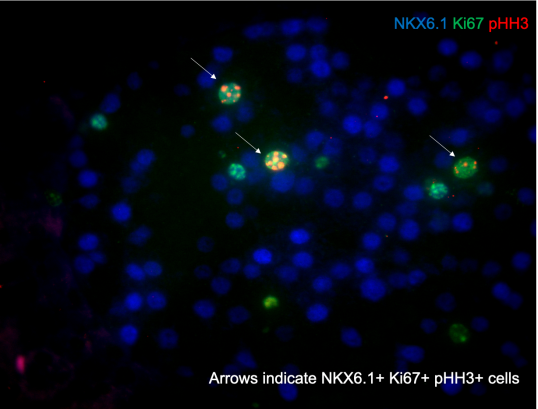
Beta cells produce insulin, a hormone that controls our energy homeostasis. Diabetes is caused by an insufficient number of beta cells or when beta cells functionally don't suffice. Beta cells intensively communicate with each other and with other cells in their microenvironment. We hypothesize that dissection of this microenvironment will allow us to generate beta cells in a dish as well as in patients as signals from the microenvironment are essential for the formation of functional beta cells during embryogenesis and postnatally. Both macrophages and endothelial cells significantly contribute to the beta cell microenvironment. We aim to understand the signals these cell types provide to the beta cells.
Therefore, we characterized a subpopulation of “regenerative” macrophages that is necessary for the formation of beta cells in experimental mice under conditions of tissue damage and regeneration (Van Gassen et al, Eur J Immunol. 2015; Van Gassen et al, Stem Cells Transl Med. 2015. Following isolation, these macrophages from an injured pancreas appeared to induce beta cell proliferation when transplanted in healthy mice. The macrophages precisely control their activities and therefore are thought no to provoke unrestrained expansion of the beta cell mass, making them good candidates for a safe therapy.
To investigate the interaction between endothelial cells and beta cells we use transgenic mice that allow conditional interruption of signal transduction by Vascular Endothelial Growth Factor (VEGF). Vessel ablation near beta cells is less dominant than generally accepted (D’Hoker et al, Diabetes. 2013; Staels et al, Diabetologia. 2017). We recently disclosed that the beta cell mass significantly increases by proliferation when endothelial cells are allowed to reappear. By adapting the microenvironment under the influence of endothelial cells, the beta cell mass can thus be augmented, a major goal in diabetes therapy. The current challenge is to disclose the mechanism behind this phenomenon and translate it into a safe therapeutic approach.
Project 3: Macrophages for beta cell regeneration and transplantation
Although macrophages are classically considered detrimental for pancreatic beta cell survival and function, recent data uncovered that alternatively activated M2 macrophages are not only able to protect beta cells during pancreatitis but also to orchestrate beta cell proliferation and regeneration after beta cell injury. The accompanying figures show massive macrophage infiltration in the pancreas tail after partial duct ligation preceding beta cell cycle activation (Van Gassen et al., Eur J Immunol, 2015).
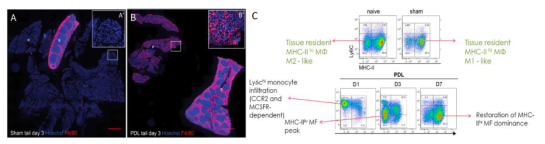
This research project aims to further elaborate on the role of M2 macrophages in beta cell regeneration and transplantation and to identify the macrophage-derived betatrophic factors in order to design novel curative strategies for diabetes.
